Aluminium is Highly Reactive Metal
But because nitric acid is an oxidizing acid the oxidizing agent is not the. Aluminium forms amphoteric oxides that is it shows both acidic and basic character.

Reactivity Series Of Metals Get The Chart Significance And Much More
Other known oxidation states are 2 and 1.

. It is often protected by a layer of inert transparent Aluminium oxide Al 2 O 3 that forms rapidly in the air but is found to be highly reactive in nature. The change of non-metallic to metallic character can be. 2H 2O and cryolite Na3AlF 6 are the important minerals of aluminium.
Since aluminium is a reactive metal it may corrode more quickly when in electrical contact with most other metals. Aluminium is the most abundant metal and the third most abundant element in the earths crust 83 by mass after oxygen 455 and Si 277. Biologically reactive aluminium is present throughout the human body and while rarely it can be acutely toxic much less is understood about chronic aluminium intoxication.
Like all alkali metals lithium is highly reactive and flammable and is stored in. The reaction between metal oxides and aluminium is highly exothermic and the metals are obtained in molten state. 7 Recycled aluminium Secondary aluminium Recycled aluminium is known as secondary aluminium but maintains the same physical properties as primary aluminium.
The reactivity series allows us to predict how metals will react. Fe 2 O 3 2Al Al 2 O 3 2Fe Heat. Cryolite Na 3 AlF 6.
Lithium is a chemical element with atomic number 3 which means there are 3 protons and 3 electrons in the atomic structureThe chemical symbol for Lithium is Li. Powder coating is a highly versatile aluminium surface treatment option and offers several advantages in any application. Rusting is an oxidation reaction.
Aluminium is a highly reactive metal belonging to the IIIA group of the periodic table. Benefiting from such reactive wetting we found that Co x B completely covered the surface of NCM as supported by the X-ray photon spectroscopy XPS data in Fig. Aluminium metal has an appearance ranging from silvery white to dull gray.
2gn fitting details in. The compounds formed by highly reactive non-metals with highly reactive metals are generally ionic because of large differences in their electronegativities. The prediction of galvanic corrosion is complex.
A more reactive metal will displace a less reactive metal from a compound. For instance powder coating is available in a huge range of colours from. In nature aluminium is found in the form of its oxide in its ores.
Recycling was a low-profile activity until the late 1960s when the. Gallium indium and thallium are less. Copper and silver will react with nitric acid.
Bauxite Al 2 O 32H 2 O. The reaction between iron oxide and aluminium produces molten iron. Powder coating aluminium.
Mean Coefficient of. In India it is found as mica in Madhya Pradesh Karnataka Orissa and Jammu. Recovery of the metal via recycling has become an important use of the aluminium industry.
Aluminiums free metal cation Alaq3 is highly biologically reactive and biologically available aluminium is non-essential and essentially toxic. The most reactive metals such as sodium will react with cold. The important ores of aluminium are.
Please consult Austral Wright Metals for specific advice. 266 x thickness in mm. Being extraordinarily chemically inert it is useful in highly reactive environments such as high pressure sodium lamps.
The ore is mechanically. Herein the question is asked as to how to diagnose. Aluminium oxide is commonly used as a catalyst for industrial processes.
Aluminium and zinc can react with water but the reaction is usually very slow unless the metal samples are specially prepared to remove the surface layer of oxide which protects the rest of the metal. Such reactions are called thermit reactions. Corundum Al 2 O 3.
Aluminium is mostly extracted from its bauxite ore. Under standard conditions it is the lightest metal and the lightest solid element. It is a soft silvery-white alkali metal.
On the other hand compounds formed between non-metals themselves are largely covalent in character because of small differences in their electronegativities. Read more about electron configuration here. This reaction is used to join rail tracks broken machine parts etc.
What Is The Reactive Series Of Metal Quora
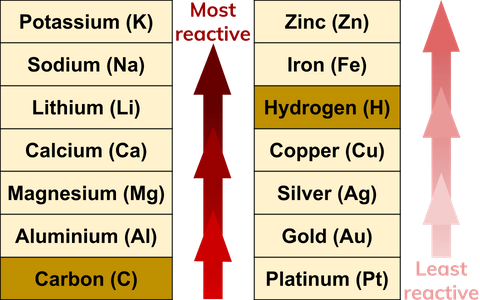
What Are Reactivity Series Definition From Seneca Learning

The Metal Reactivity Series Compound Interest
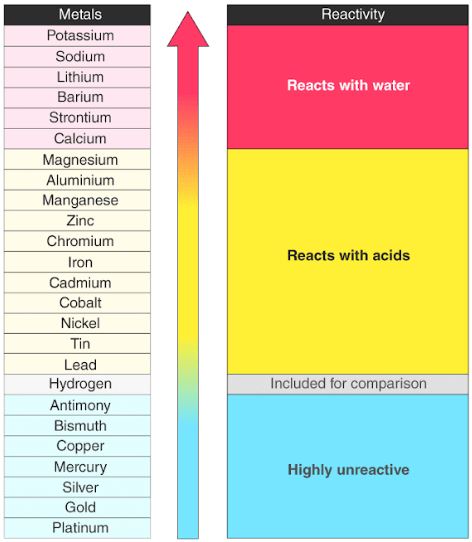
Reactivity Series Reactivity Of Metals Chart Features Uses
Which Metal Is More Reactive Aluminium Or Iron And Why Quora
0 Response to "Aluminium is Highly Reactive Metal"
Post a Comment